Targeted Delivery
Extroducer®
The Extroducer has been designed to be a minimally invasive therapeutic delivery device that is applicable to a diverse class of therapeutics including small molecules, biologics, and cell & gene therapies. Developed at the Karolinska Institute, it uses an inverted Seldinger technique for precise access to target tissues via blood vessels and is designed to reduce risks such as hemorrhage and thrombosis.
Extroducer is 510(k) cleared for applications in the abdomen and is currently in clinical trials as an investigational device for other organs.
Advantages
Many drug delivery systems are designed for systemic delivery which can lead to suboptimal drug concentrations in the target tissue, off-target toxicity, or low retention of the drug in target tissues. A minimally invasive endovascular infusion catheter, the Extroducer is designed to provide direct-to-tissue and direct-to-tumor therapeutic access using conventional surgical setup and software, lowering the barrier to entry for hospitals and surgeons as well as clinical and pre-clinical collaborators.
Design Features
Expected Benefits
Elegant design
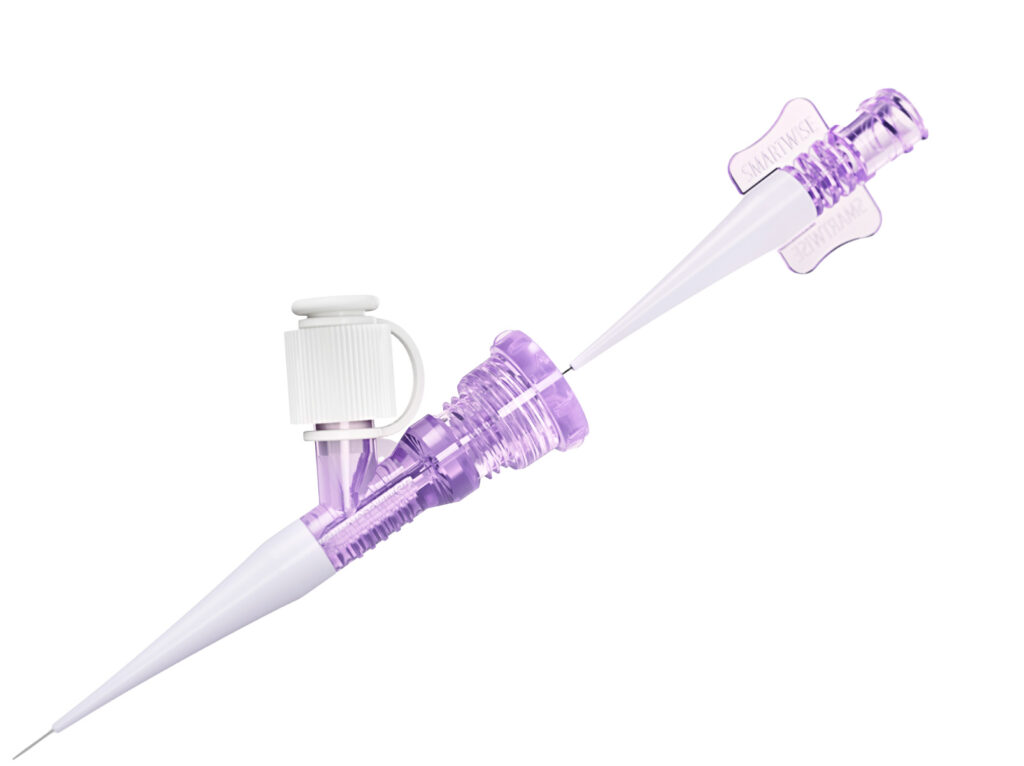
The Extroducer has been designed to enhance tissue retention of drug, reduce systemic toxicity by only delivering the therapeutic where it is needed the most, and allow safe, repeated access to challenging target tissues with minimal invasiveness. The Extroducer device consists of three key components.
01Microcatheter
The Extroducer is compatible with most off-the-shelf micro catheters
02Protective sheath
The protective sheath enables precise maneuvering of the sharp needle within the vasculature to the target tissue.
03Needle collar
The radiopaque needle collar is depth limiting for added safety, preventing over-penetration of the needle.
04Infusion needle
The infusion needle includes proprietary sharpening of the tip which allows for efficient and minimally invasive injection of payload into tissue while limiting risk of adverse events. The sharp tip is intended to create a self-sealing working channel through the vessel wall.
Use cases
With the ability to deliver many types of therapeutic payload, the Extroducer has numerous potential clinical indications. Whether the goal is to access challenging or risky target tissue locations or to enhance therapeutic efficacy through direct-to-target delivery, the Extroducer has been designed to improve patient outcomes in diverse clinical settings.
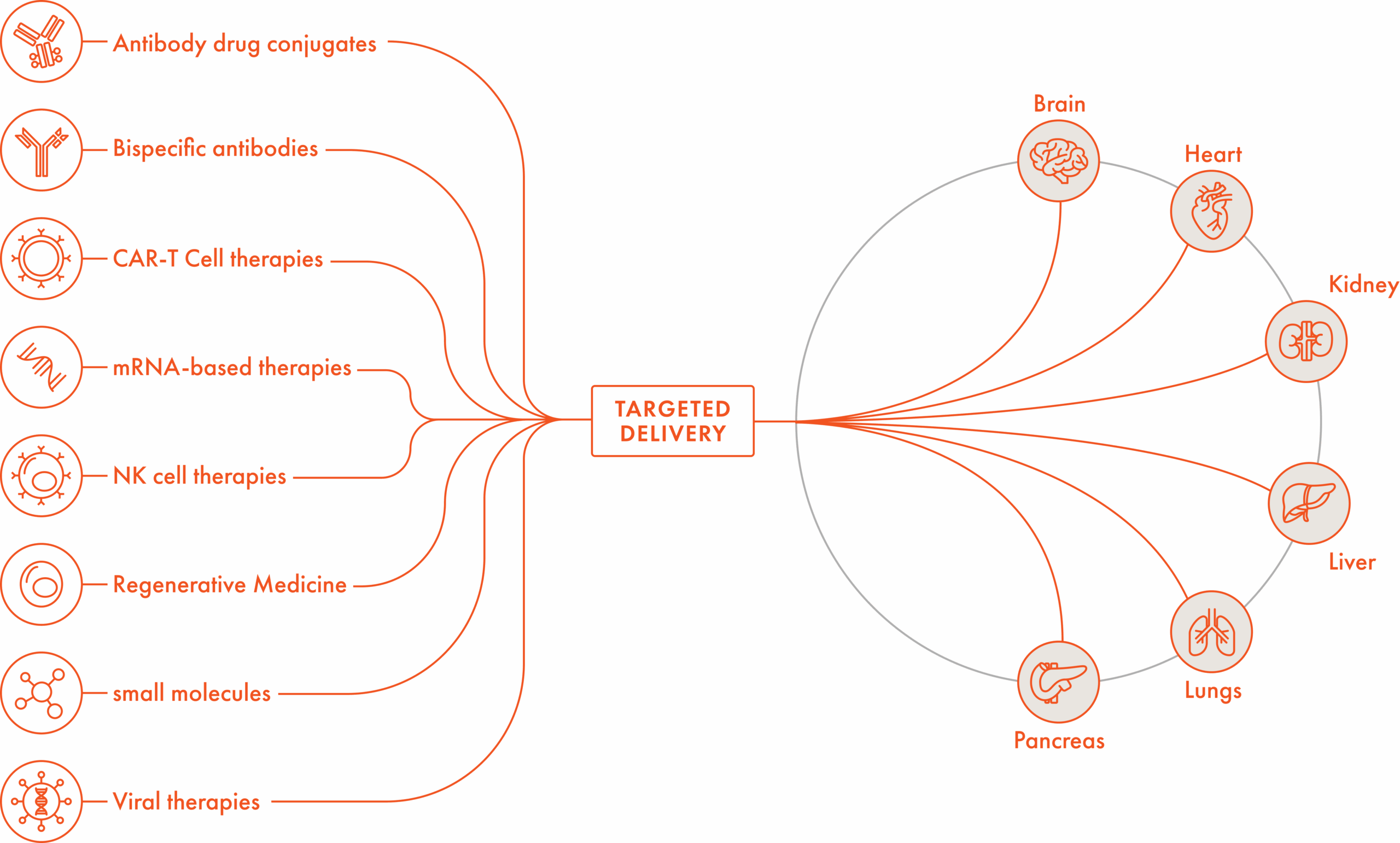
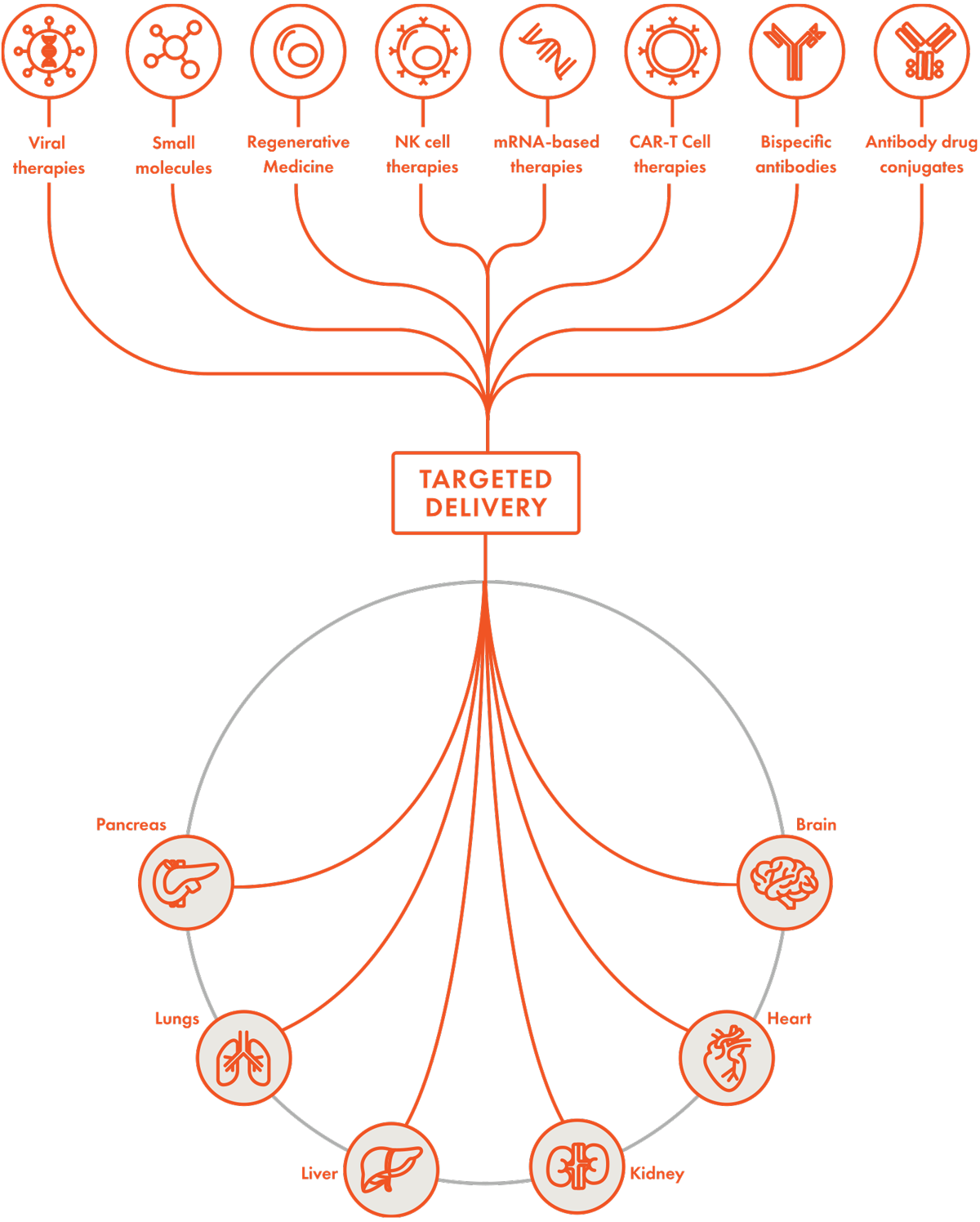
The Extroducer has the potential to effectively deliver CAR-T cell therapies, antibody drug conjugates, bispecific antibodies, NK cell therapies, small molecules, viral therapies, mRNA therapies and regenerative medicines. Potential applications of the Extroducer could include the above (all of which would require clinical testing for regulatory clearance).
Regulatory details
The initial version of the Extroducer was 510K cleared by the FDA in 2022 for abdominal organs, with a 510(k) submission projected for an updated version in Q4 2025. Pre-clinical work is ongoing for a number of other applications including heart, pancreas, kidney, liver, and lung.
Submission projected Q4 2025
Preclinical studies
Pre-clinical studies show the Extroducer is effective for regenerative therapies such as delivering human ventricular progenitor (HVP) cells and viral gene therapies (Ad5) to repair damaged heart tissue.
Other tested cardiac payloads include modRNA, AAV, and cell therapies, all demonstrating high accuracy and retention.
Potential benefits include:
Increased retention
Reduced risk of pericardial effusion compared to conventional devices
Manageable safety profile for arrhythmia and other complications
Explore recent breakthroughs and industry trends

Interested in using the Extroducer for your next delivery?
